Bioenergetics Growth Model for the Effect of Gamma Irradiation and/or Plant Extract Barnoof on the Progeny of Black Cutworm, Agrotis ipsilon (Hufngel) 
 Author
Author  Correspondence author
Correspondence author
International Journal of Molecular Zoology, 2012, Vol. 2, No. 2 doi: 10.5376/ijmz.2012.02.0002
Received: 19 Apr., 2012 Accepted: 23 Apr., 2012 Published: 11 May, 2012
Mohamed, 2012, Bioenergetics Growth Model for the Effect of Gamma Irradiation and/Or Plant Extract Barnoof on the Progeny of Black Cutworm, Agrotis Ipsilon (Hufngel), International Journal of Molecular Zoology, Vol.2, No.2 13-22 (doi: 10.5376/ijmz.2012.02.0002)
In this study, we investigate the effects of two substerilizing doses 100 and 150 Gray (Gy) of gamma irradiation applied to Full grown pupae of the Black cutworm, Agrotis ipsilon (Hufngel). and/or plant extract, Conyza dioscorides (Barnoof) with two different solvents and applied to the resulting 5th-6th instar larvae in F1 generation on certain biological aspects and the energy budget.
Data revealed that gamma irradiation when combined with the extract in most treatments significantly higher than the control of reproduction at all treatments (doses and concentrations).
Most of the treatments increased the values of developmental/day, larval duration and % survival were measured. These indices in the F1 progeny as compare to the control treatment, except, at the % survival it was decreased at all treatments. These values were more obvious in combination treatments compared to gamma irradiation or plant extract treatments each of them alone.
The coefficient of metabolizable energy (C.M.E.) not affected at all treatments. The efficiency of storage of ingested energy [E.S.I.(E)] and the efficiency of storage of metabolizable energy [E.S.M.(E)] were between increasing and decreasing when the F1 progeny treated with plant extract alone or combined with the dose 100 Gy while it were significantly increased at all treatments when the dose 150 Gy combined with the plant extract. The results obtained were discussed in terms of their implications for the best substerilizing dose for the irradiation of parental male pupae of A. ipsilon combined with the dose 150 Gy.
Among the lepidopterous species, the Black cutworm, Agrotis ipsilon (Hufnagel) is one of the most severe insect pests in Egypt. The release of sterile males for the control of natural insect populations has not had great success with species of Lepidopteron, because they are highly resistant to irradiation (North and Holt, 1968 and LaChance et al., 1973) Several reports on Lepidoptera stated a mounting success in the use of gamma radiation at low doses to face a successful inherited sterility (Proverbs et al., 1975; Carpenter et al., 1987).
Several plant extracts and/or their lonely active compounds have demonstrated extensive potential as acute or chronic insecticides, insect growth regulators, or antifeedants against a Varity of insect species (e.g. Beckage et al., 1988, Jilani and Saxema, 1990). Such feeding deterrence and/or growth regulation may be correlated with the magnitude of biochemical changes in the test species.
However, the combined effects of gamma radiation and bioinsecticides on Lepidopterous insects were studied by several authors, Sallam et al (1991); Mohamed (2004); Mohamed et al (2004); EL-Nagar et al (2004); El-shall and Mohamed (2005) and Mohamed (2006).El-Nagar (1990) found that the inherited sterility of the spodoptera littoralis was successfully achieved when full grown pupae were substerilized with 10 K, 15 K and 20 K rad of gamma radiation.
The present study was undertaken to elucidate the energy budget of 5th~6th instar larvae of the Agrotisipsilon treated with plant extract Barnoof which resulting from irradiated parental male full grown pupae with the two substerilizing doses 100 Gy or 150 Gy, i.e. energy budget of F1 progeny. Energy budget is useful in understanding the basic nutritional ecology, with sufficient comparisons among various individuals, species and population, thus indicating the performance of insects in terms of rates of production and food consumption (Ananthakrishnan, 1994).
1 Results and Discussion
The reproductive behavior of A. ipsilon treated as full grown male pupae was observed among P1 and F1 generation (Figure 1). The adverse effect of irradiation on the reproductive potential was found to be greater for females than for males, these results agreement with Knipling (1970), Carpenter et al (1986) and Mohamed (2004), who mentioned that the females were more radiosensitive than males as determined in the reduction in fecundity and egg viability.
 Figure 1 Effect of gamma irradiation of parental male pupae on reproduction among P1 and F1 |
The effect of plant extract Barnoof on the reproductive behavior among F1 generation descendant of P1 (N♂×N♀) was observed in Figure 2, which shows that the adverse effect of plant extract Barnoof on the reproductive potential was found to be greater for females than for males when F1 progeny treated with acetone extract at all treatments but it was not among F1 progeny treated with petroleum ether extract, while sterility was greater for females than for males at the treatments 1.5% and 3% petroleum ether extract. The effect of plant extract Barnoof applied to F1 progeny descendant of irradiated parental male pupae with the dose 100 Gy on reproduction of A. ipsilon was shown in Figure 3. The data indicate also the adverse effect of the combination between irradiation and plant extract on the reproductive potential was found to be greater for males. These results agreement with EL-Naggar et al (2004), Mohamed (2004) and El-shall and Mohamed (2005).
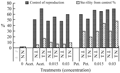 Figure 2 Effect of plant extract Barnoof on reproduction among F1 generations of A. ipsilon adults descendant of P1 (NMXNF) |
The effect of plant extract Barnoof applied among F1 progeny descendant of irradiated parental male pupae with the dose 150 Gy on reproduction of A. ipsilon was shown in Figure 4. Data shows a Slide affect in case of acetone extract but found the adverse effect of the combination between irradiation and plant extract on the reproductive potential greater for females than for males among the petroleum ether extract treatments, this agreement with the previous authors.
Originally, the radiosensitivity of male A. ipsilon regarding inherited sterility was assessed with revere to the combination between 100 and 150 Gy of gamma radiation and plant extract Barnoof among F1 progeny. The male moth descendant of irradiated parental male pupae were found to be better candidates for producing more inherited sterility among F1 progeny (Figure 3; Figure 4). It was essential to study the F1 viability in relation to growth, food utilization and energy efficiencies of the F1 larval stage, which might reflect the behavioral potency of F1 adults.
 Figure 3 Effect of plant extract Barnoof applied to 4th~5th instar larvae among F1 generation descendant of irradiated parental male pupae with the dose 100 Gray on reproduction of A. ipsilon adults |
Figure 5 shows the various growth and development characteristics of A. ipsilon in the F1 progeny of treated males, it shows the effect of gamma irradiation alone or in combination with acetone or petroleum ether Barnoof extract on larval duration, % survival and % development per day among F1 progeny. Most treatments decreased the values of these indices of the F1 progeny, as compared to the untreated control. Thereduction in these values was more obvious in combination treatments compared to gamma irradiation or plant extract treatments each of them alone. However, this decrease in the values of the previous indices associated with an increase of assimilation index (Figure 6) in most treatments compare to control. On the other hand, a significant increase of the assimilation index values was observed at the majority of combination treatment as compare to the untreated control (El-Shall and Mohamed, 2005).
 Figure 4 Effect of plant extract Barnoof applied to 4th~5th instar larvae among F1 generation descendant of irradiated parental male pupae with the dose 150 Gy on reproduction of A. ipsilon adults |
Figure 5 The effect of gamma irradiation alone or in combination with acetone or petroleum ether Bamoof extract on % development/day, larval duration and % survival to the nutritional profile of A. ipsilon extract on % development/day, larval duration and % survival to the nutritional profile of A. ipsilon among F1 progeny |
 Figure 6 The effect of gamma irradiation alone or in combination with acetone or petroleum ether Bamoof extract on Growth index, Survival index and Assimilation index to the nutritional profile of A. ipsilon among F1 progeny |
The post-embryonic developmental period of F1 progeny was delayed, resulting in a reduction in the growth index, which exhibited a negative relationship with the gamma dosage only and when it combination with the plant extract.
The gross energy (or heat of combustion) of a substance is defined as the energy liberated as heat when the substance is completely oxidized to CO2 and H2O (Kleiber, 1961; Tyler, 1964).
The influence of the two substerilizing doses (100 and 150 Gy) on the dry matter nutritional profile, as a function of the physiological response to irradiation and/or irradiation combined with plant extract stress among F1 progeny was studied in our previous papers.
Table 1 indicates the effects of Barnoof extract alone on the energy budget of the F1 progeny of A. ipsilon. The gross energy in food balance (digested) of F1 progeny showed a negative correlation with plant extract, which resulted in a significant reduction in energy in food balance (digested) up to the levels 68.98%, 71.03% and 72.38% from the control at 0%, 1.5% and 3% respectively to the acetone extract and 81.22%, 78.50% and 74.15% at 0%, 1.5% and 3% respectively of petroleum ether extract among F1 progeny, this decrease might be attributed to the lowest food intake by treated the larvae with the plant extract, whereas, the plant extract Barnoof combined with 100 Gy of gamma radiation (Table 2) increased the gross energy in food digested (food balance) to 85.99%, 87.62% and 88.71% from the control treatment in case of acetone extract (0%, 1.5% and 3%) respectively. Then increased to 82.45%, 87.21% and 90.88% from the control treatment in case of the petroleum ether extract (0%, 1.5% and 3%) respectively, except, at the 100 Gy treatment only still significantly decrease to 69.12% from the control treatment. The gross energy in food digested (food balance) was significantly decreased to 66.80 % from the control treatment at the dose level 150 Gy (Table 3). The dose level 150 Gy combined with the acetone extract (Table 3 also) decreased the gross energy in food digested (food balance) to 84.76% and 66.53% from the control treatment in 1.5% and 3% acetone Barnoof extract (combined with the dose level 150 Gy). Furthermore, the combination between the dose level 150 Gy and the concentration 3% petroleum ether extract treatment significantly decrease the gross energy in food digested to 79.91% from the control treatment. Then, the great effect was in the higher dose (150 Gy) combined with the higher concentration (3%) of plant extract Barnoof (Mohamed, 2004; El-Nagar et al., 2004; Mohamed et al., 2004; El-shall and Mohamed, 2005; Mohamed, 2006).
 Table 1 Effect of acetone and petroleum ether Barnoof extracts on the energy budget of A. ipsilon in F1 progeny |
The energy stored in the body, the coefficient of metabolizable energy (CME), the efficiency of storage of ingested energy [ESI(E)] and the efficiency of storage metabolizable energy [ESM(E)] were determined inTable 1, Table 2 and Table 3.
Table 1 shows that the gross energy stored in the bodies of F1 progeny significantly decreased at all treatments to 52.79%, 76.45% and 60.17% from the control treatment at the concentrations 0%, 1.5%, 3% of acetone extracts respectively and significantly decreased to 73.13%, 59.21% and 62.02% from the control treatment at the concentrations 0%, 1.5% and 3 % of the petroleum ether extracts respectively. These previous levels or data exceed or more significantly increased when plant extract combined with the dose rate 100 Gy in Table 2 and also with the dose level 150 Gy.
In most treatments on Table 3, the significantly increase in the gross energy stored in the bodies of F1 progeny higher from 52.79% from the control treatment in acetone treatment Table 1 to 83.86% from the control treatment when acetone only combined with both dose levels 100 and 150 Gy (Table 2 and Table 3). The gross energy stored in the bodies significantly increased from 76.45% and 60.17% in 1.5% and 3% acetone extract in Table 1 to 83.86% and 70.35 % from the control treatment when combined with the dose level 100 Gy respectively in Table 2 and increased to 136.89% and 123.86% from the control treatment respectively when combined with the dose level 150 Gy in Table 3. Also, increased from 59.21% and 62.02% from the control treatment at the concentrations 1.5% and 3% of petroleum ether extract to 91.76% and 92.13% from the control treatment when combined with the dose level 100 Gy in Table 2 and increased to 112.85% and 118.37% from the control treatment when combined with the dose level 150 Gy. Then, the combined of gamma irradiation with plant extract Barnoof cause the increasing in the gross energy stored in body, these results agreement with Shaurub et al (2001) and disagreement with Seth and Sehgal (1992). Generally, the energy stored in the bodies of treated F1 progeny with plant extract was lower than that stored in the bodies of non-treated larvae (ones) than it was higher when combined with 100 Gy and 150 Gy of gamma irradiation, this might be due to the ability of treated larvae to utilize efficiently the input energy as indicated by highest ESI (E) and ESM (E) relative to the non-treated ones (Table 2; Table 3).
 Table 2 Effect of gamma irradiation (100 Gy) alone or in combination with Barnoof extract on the energy budget of A. ipsilon in F1 progeny |
 Table 3 Effect of gamma irradiation (150 Gy) alone or in combination with Barnoof extract on the energy budget of A. ipsilon in F1 progeny |
Gross energy stored in the bodies to the most treatment of 5th and 6th instar larvae among F1 progeny treated with plant extract Barnoof which descendant of irradiated parental male pupae with 100 Gy and 150 Gy (Tables 2; Tables 3) higher than that stored in the bodies of the larvae treated with the plant extract only (Table 1).This large amount of energy is, of course, needed to support the non-feeding pupae, as has been pointed out by Waldbauer (1968) and Shaurub et al (2001).
After absorption of the digested food, some of the compounds are used for the metabolic energy and a certain proportion is converted into biomass. Thus, the coincidence of the pattern of plant extract and/or irradiation impact on the metabolizable energy (C.M.E.) and on the gross energy in food digested (food balance) of either treated with plant extract and/or irradiated larvae of F1 progeny was expected.
In this situation, gamma irradiation combined with plant extract Barnoof did not affect on the C.M.E. at all treatments (Table 1, Table 2 and Table 3).
Acetone only significantly decreased the efficiency of storage of ingested energy [E.S.I. (E)] and the efficiency of storage or metabolizable energy [E.S.M. (E)] to 80.69% and 78.69% from the control treatment respectively, while the acetone extract (1.5% concentration) significantly increased to 116.66% and 112.13 % from the control treatment respectively. The petroleum ether extract (1.5% concentration) significantly decreased the [(E.S.I. (E)] and [E.S.M. (E)] to 78.37% and 76.91% from the control treatment respectively (Table 1).
Table 2 indicate that the [(E.S.I. (E)] and [E.S.M. (E)] significantly increased to 151.24% and 155.93% respectively from the control treatment among F1 larvae descendant of irradiated parental male pupae with 100 Gy. Also, significantly increased to 149.44% and 145.85% from the untreated control respectively among F1 larvae treated with 3% acetone extract combined with 100 Gy.
Data in Table 2 also indicate that the combination of gamma irradiation and petroleum ether extract did not affected on both [(E.S.I. (E)] and [E.S.M. (E)] at the most treatments, while significantly increased at all treatments in Table 3. These results are disagreement with those obtained by Seth and Sehgal (1992), they mentioned that C.M.E., E.S.I.(E) and E.S.M.(E) of 6th instar larvae of Spodoptera litura were generally decreased with increasing the dose of gamma radiation, and agreement with Thornburn (1972) who reported that gamma irradiation causes interruption of energy supplies and blocking of key enzymes which may stop normal metabolism. Also agreement with Shaurub et al (2001), who mentioned that irradiation with doses 10, 15 and 20 krad increased C.M.E., E.S.I. (E) and E.S.M. (E) in case of 6th instar larvae. Nestel et al (1986) concluded that there was a correlation between the energetic balance of irradiated Ceratitis capitata adults and the lipid metabolism. Reynolds and Nottingham (1985) reported that the decrease in E.C.I. and E.C.D. is attributed to increased total metabolic costs (from the energy budget), i.e. the metabolic costs increased relative to energetic input. These costs are likely of respiratory nature (Reynolds and Nottingham, 1985; Chaabane et al., 1999). Therefore, it appears that E.S.I. (E) and E.S.M. (E) are overall measures of the insect's ability to utilize the energy in food ingested and digested respectively.
Consequently, E.S.I. (E) and E.S.M. (E) are considered as the gross and net energy utilization efficiencies, respectively for the maintenance of life. The previous findings indicate that energy utilization efficiencies incase. These data agreement with Shaurub et al (2001) they pointed out that the importance of optimization of the dose in irradiation of any insect species. Therefore, the dose 20 krad appears to be the optimal dose for irradiation of S. littoralis pupae, where at this dose level the energy utilization efficiencies [E.S.I.(E)] and [E.S.M.(E)] together with the energy stored in the body were drastically decreased in both 4th and 6th instar larvae resulting from irradiation of the parent pupae of this insect species.
Food materials are not used directly in the body. Instead they are converted into a potential (stored) energy in the form of adenosine triphosphate (ATP) which is then used to perform the metabolic processes. During these processes, energy is released (exergonic or catabolic reaction) or absorbed (endergonic or anabolic reaction), converted to heat and dispersal into environment. Cells have no way of producing new energy or of recycling the energy they have used. Thus, life depends on a continuous in put of energy. This energy flows in one way direction through every cell and organism, and through the ecosphere. Accordingly, an organism is considered as open system with respect to energy. While, the insects are a major component of almost terrestrial or fresh water communities; very little appreciation has been paid for studying their energetic (Shaurub et al., 2001).
Finally, it could be concluded that the SIT depending on inherited sterility is usually not a “stand alone” technology, but also, a complementary component of an area wide and sustainable long-term strategy in Integrated Pest Management (IPM) (El-shall and Mohamed, 2005). Compatible control methods, such as plant extracts, that possess a source for naturally occurring substances acting, as feeding and insect growth deterrents may be synergistic when integrated with the SIT. Since, the present data indicated that the combination treatment of irradiation and Barnoof extracts resulted in less consumed food and more food utilization efficiency compare to the use of irradiation alone. In addition the reduction of growth index due to combined treatments would lead to the emerging of undersized female moths. These females may lay fewer eggs than female resulting from irradiation treatments only (Mohamed, 2004). Furthermore, the fertility is a fundamental parameter in the assessment of any control measure. The combination treatments of Barnoof extracts and gamma irradiation on A. ipsilon decreased the fertility of moths less than that resulted from the irradiation treatments alone. Further critical evaluations need to be conduct; both in the laboratory and in the field, on the effect of gamma irradiation and/or plant extract for assessment the use of plant extracts in suppress the pest population before introducing the SIT.
2 Materials and Methods
The laboratory strain of A. ipsilon reared on castor leaves, Ricinus communis at 26℃-28℃ and 60±5% R.H. The source of gamma radiation used during the present study was from a Cobalt 60(60Co) irradiator; the dose rate of irradiation source was 145 rad/min.
The full-grown male pupae of A. ipsilon were irradiated 24-48 hours before adult emergence (male line) with low doses of gamma radiation (100 Gy; 150 Gy) in a 100cc carton cups. After adult emergence, fifteen groups of each dose (1 treated male×1 normal female) were made in each treatment. A group of fifteen untreated pupae were also kept as a control (N♂×N♀). Each pair (male and female) was kept in 750cc cylindrical glass cage supplied with 10% sugar solution. The daily-deposited eggs of non-irradiated females were collected, counted, recorded and kept for calculating the % of egg hatch. The sterility from control and control of reproduction were computed (as follow) in response to the reproductive performance of the treated parental insects and also in their F1 progeny involved in different treatments. Control of reproduction given by (Vc-V t) / Vc *100 where:
Vc = viable number of eggs in the control
Vt = the viable number of eggs in the treated insects (Seth and Shegal, 1992).
To continue the F1 generation for male line, newly hatched larvae resulting from irradiated P1 males were kept in groups in glass jars provided with castor bean leaves. Each four hundred larvae of the second instar of each treatment were transferred to a glass jar (500cc), furnished with a thin layer of saw dust to absorb excess humidity and covered with muslin fixed with a rubber band. Rearing was continued in the same way until the larvae reached the last larval instar, then were transferred individually to small plastic vials (each, 10cc) containing moistened saw dust for pupation. Newly formed pupae were collected, sexed and each sex was kept separately in a plastic container with moistened sawdust and covered as usual with muslin cloth. At each treatment, the newly emerged males of the first generation (F1) descendant of irradiated parental males were paired with newly emerged untreated females using all possible crosses between them in order to obtain F1 generation. The crossing scheme for this study is illustrated as follows:
P1 Crosses: (Normal male×Normal female) serve as a control [Treated male×Normal female (male line)]
F1 Crosses: (Normal male×Normal female) serve as a control
F1 Male (male line)×Normal female
[Normal male×F1 Female (male line)]
The Barnoof, Conyza dioscorides leaves were drying at room temperature for 2~3 weeks, and then pulverized into a fine powder with an electric mill. The powdered plant material extracted successively with petroleum ether or acetone solvent, as described by Freedman et al (1979). The extracts obtained were weighted and re-dissolved in the both solvents to obtain 10% stock solutions (w/v), which were stored under refrigeration until needed. 0%, 1.5% and 3% of the Conyza dioscorides plant extract with the both solvents, were selected to treat the newly moulted fourth-instars larvae of F1 progeny. The castor leaves were sprayed with different dilutions of plant extracts, and then the leaves were left until dry. The irradiated and non-irradiated larvae were fed on the treated leaves for 24 hours.
For consumption and utilization of food studies, three experimental groups were set up. The first group consists of the progeny of F1 larvae irradiated as parental full-grown male pupae with 100 Gy and 150 Gy. The second one consists of the progeny of F1 larvae irradiated as parental male pupae with the same each previous dose and treated as fourth instar with each of the two concentration of the plant extract for each solvent. The third group consists of larvae treated with plant extract only with each two concentration and solvents. A parallel group of untreated insects used as control. Twenty Larvae were put in glass jar for each treatment. Each experimental group was replicated three times. Food consumption and utilization were estimated during the fifth and sixth instars larvae for six days.
Food utilized by larvae calculated according to the equation denoted by Waldbauer (1968). Consumption Index (C.I.), Growth Rate (G.R.), Approximate Digestibility (A.D.), Efficiency of Conversion of Ingested food (E.C.I.) and Efficiency of Conversion of Digested food (E.C.D.) were calculated as follows:
C.I.=F/TA G.R.=G/TA
Where F=weight of food eaten
G=weight gain of insect during feeding period
T=duration of feeding period
A=initial weight of insect+Final weight of insect/2 A.D.=(weight of food ingested-weight of faces)/weight of food ingested*100
E.C.I.=weight gained/weight of food ingested*100 E.C.D=weight gained/(weight of food ingested-weight of faces)*100
The combined effect of gamma irradiation and/or plant extract on developmental, survival and assimilation indices of larvae of F1 progeny were calculated as follows:
1. % Developmental/day=100/average larval duration (days) (Power and Oatman, 1984)
2. Growth index=% survival/average larval duration (days) (Moonis, 1979).
3. Survival index=% survival/Maximum % survival in all treatments (Moonis, 1979)
4. Assimilation index=% development/day×Live weight (grams) (El–Sherif et al., 1996; El–Sherif, 1998)
The rate of change in both metabolic and developmental parameters of treated insects was calculated as percentage from the control (I/C).
Data were analyzed using the Analysis of Variance (ANOVA) technique and the means were separated using Duncan×™s multiple range test (P> 0.05) (Steel and Torrie, 1980).
References
Ananthakrishnan T.N., 1994, Functional dynamics of phytophagous insects, Oxford & IBH Publishing Co. PVT. Ltd., New Delhi, Bombay & Calcutta, pp.304
Beckag N.E., Metcalf J.S., Neilsen B.D., and Nesbit D.J., 1988, Disruptive effects of azadirachtin on development of Cotesia congregata in host tobacco budworm larvae, Arch Insect Biochem. Physiol., 9: 47
http://dx.doi.org/10.1002/arch.940090104
Carpenter J.E., Young J.R., and Sparks A.N., 1986, Full army worm, Spodoptera frugiperda (Lepidoptera-Noctuidae): Comparison of inherited deleterious effects in progeny from irradiated males and females, J. Econ. Entomol., 79: 46
Carpenter J.E., Young J.R., Sparks A.N., Cromroy H.L., and Chowdhury M.A., 1987, Corn earworm (Lepidoptera: Noctuidae): Effects of substerilizing doses of radiation and inherited sterility on reproduction, J. Econ. Entomol., 80: 483
Chaabane K., Josens G., and Loreau M., 1999, Respiration of Abax ater (Coleoptera,Carabidae): a complex parameter of the energy budget, Pedobiologia, 43:305-318
El-Naggar, S.E.M. 1990. Biological and histological studies in the progeny of gamma-irradiated cotton leafworm, Spodoptera littoralis. Ph.D.Thesis, Faculity of Agriculture (Moshtohor), Zagazig University, Egypt, pp.76
El-Naggar S.E.M., Ibrahim S.M., and Mohamed H.F., 2004, Combined and separate effects of gamma irradiation and barnoof plant extract on the dietary profile of the black cutworm, Agrotis ipsilon (Hufn.) I-Treatment of the eight days-old larvae, Seventh Arab Conference on the peaceful Applications of Atomic Energy, 4-8 Dec., Sanna, Yemen
El-Shall S.S.A., and Mohamed H.F., 2005, The combined effect of gamma irradiation and plant extract (Barnoof) on the nutritional profile of the Black cutworm, Agrotis ipsilon (Hufn.) (Lepidoptera: Noctuidae), II-The effect on the F1 progeny during the 5th and 6th instars larvae, Arab J. Nucl. Sci. Appl., 38(2): 289
El-Shazly M.M., Nassar M.I., and El Sherief H.A., 1996, Toxic effect of ethanolic extract of Nerium oleander (Apocynaceae) leaves against different developmental stages of Muscina stabulans (Diptera), J. Egypt. Soc. Parasitol., 26(2): 461-473
PMid:8754654
El-Sherif H.A., 1998, Effect of JHAs on the biology, morphology and physiology of the grey flesh fly Parasarcophaga argyrostoma (Rabineau - Desvoidy), M.Sc. Thesis, Fac. Sci. Cairo Univ.
Freedman B., Nowak J., Kwolek W.F., and Berry E.C., 1979, Abioassay for plant derived pest control agents using the European cornborer, J. Econ. Entomol., 72: 541-545
PMid:528746
Jilani R., and Saxena R.C., 1990, Repellent and feeding deterrent effects of turmeric oil, sweetflag oil, neem oil, and a neem-based Insecticide against lesser grain borer (Coleoptera: Bostrychidae), J. Econ. Entomol., 83: 629
Kleiber M., 1961, The fire of life, an introduction to animal energetic, John Wiley & Sons Inc., New York, pp.454
Knipling E.F., 1970, Suppression of pest Lepidoptera by releasing partial sterile males. A theoretical appraisal, Bioscience, 20: 465
http://dx.doi.org/10.2307/1295155
LaChance L.E., Bell R.A., and Richared R.D., 1973, Effect of Low Doses of Gamma Irradiation on Reproduction of Male Pink Bollworms and Their F1 Progeny, Environ. Entomol., 2: 653
Mohamed H.F., 2004, Inherited sterility induced by gamma irradiation and/or Barnoof plant extract on reproductive potential and mating ability of the black cutworm, Agrotis ipsilon (Hufngel) (Lepidoptera: Noctuidae), Isotope & Rad. Res., 36(4): 713
Mohamed H.F., 2006, Effects of gamma irradiation and leaves extract of barnoof plant on larval development of Agrotis ipsilon (Hufngel), Arab J. Nucl. Sci. Appl., 39(2): 255
Mohamed H.F., EL-Naggar S.E.M., Mohamed A.Z., 2004, The combined effect of gamma irradiation and plant extract barnoof on the nutritional profile to the black cutworm, Agrotis ipsilon, III. The effect on some haemolymph digestive enzyme activities, J. Egypt Acad. Soc. Environ. Develop. (C-Molecular Biology), 5(2): 99
Moonis A.J., 1979, Studies on food and feeding habits of Triophidia ammlate (Thunberg) (Orthoptera: acrididae), Ph.D. Thesis, Department of Zoology Aligarh Muslim University, India
Nestel D., Galun R., and Friedman S., 1986, Energetic balance in irradiated Ceratitis capitata (Weid.) adults (Diptera: Tephritidae), Folia Entomol. Mexic., 70: 75-85
North D.T., and Holt G.G., 1968, Genetic and cytogenetic basis of radiation-induced sterility in the adult male cabbage looper Trichoplusia ni. Isotopes and Radiation in Entomology, Proc. Symp.Vienna, 1967, IAEA, Vienna
North D.T., and Holt G.G., 1968, Inherited sterility in progeny of irradiated male cabbage loopers, J. Econ. Entomol., 61: 928
Power N.R., and Oatman E.R., 1984, Biology and temperature responses of Chelonus kellieae and Chelonus phthorimaeae (Hymenoptera: Braconidae) and their host, the potato tuberworm, Phthorimaeae operculella (Lepidoptera: Gelechiidae) [morphology, adult food, host age, mating, oviposition], Hilgardia, 52:1
Proverbs M.D., Newton J.R., Logan D.M., and Brinton F.E., 1975, Codling moth control by release of radiation-sterilized moths in a pome fruit orchard and observations of other pests, J. Econ. Entomol., 68: 555
Reynolds S.E., and Nottinghan S.F., 1991, Effects of temperature on growth and efficiency of food utilization in fifth-instar caterpillars of the tobacco hornworm, Manduca sexta, J. Insect physiol., 31- 129
http://dx.doi.org/10.1016/0022-1910(85)90017-4
Sallam, H.A., Elnagar S., and Ibrahim, S.M., 1991, Effects of gamma radiation on reproductive potential of the black cut worm, Agrotisipsilion (Hufn.), Arab J. Nucl. Sci. and Appl., 24: 165-174
Seth R.K., and Sehgal, S.S., 1992, "Growth, bioenergetics and reproductive Competence "In" Management of insect pests nuclear and related molecular and genetic technology" Proc. Symp., Vienna, 13-23 October, IAEA, SIT/ bubl 909, pp.427-440
Shaurub E.H., EL-Naggar S.S.M., and EL-Shall S.S.A., 2001, Effect of substerilizing doses of gamma radiation on the economy of bioenergetics of the cotton leaf worm, Spodoptera littoralis (Boisd.) (Lepidoptera : Noctuidae), J. Egypt Ger. Soc. Zool., 34(E): 19
Steel R.G.D., and Torrie J.H., 1980, "Principle and procedures of 2nd. ed., McGraw-Hill book Co., New York
Thornburn C.C., 1972, Tsotopes and radiation in biology. Butterforth & Co., Ltd., London
Tyler C.C., 1964, Animal nutrition, Chapman & Hall, London, pp.253
Waldbauer G.P., 1968, The consumption and utilization of food by insects, Adv. Insect Physiol., 5: 229 http://dx.doi.org/10.1016/S0065-2806(08)60230-1
. PDF(805KB)
. FPDF(win)
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. Hussein Farid Mohamed
Related articles
. Gamma radiation
. Agrotis ipsilon
. Plant extract
. Reproductive potential
. Bioenergetics
Tools
. Email to a friend
. Post a comment


